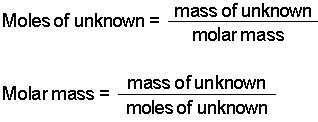

Here is an example which we will go through at the start of the lesson, and which will be easy for you if you've read this first:
What mass of calcium hycroxide do we need to neutralise 146g of hydrochloric acid?
First we need a balanced symbol equation:
2HCl + Ca(OH)2 > CaCl2 + 2H2O
This tells us that one mole of calcium hydroxide will react with two moles of HCl (because the balanced equation has 2HCl but only one Ca(OH)2 )
So how many moles of HCl do we have? This is the mass divided by the molar mass, so 146 / (1+35/5) = 4 mol.
So how many moles of Ca(OH)2 do we need? One will react with two moles of HCl, so we will need 2 to react with 4.
What is the mass of Ca(OH)2 needed? This is given by mass = moles x formula mass = 2 x (40+(2 x (16+1))) = 2 x 74 = 148g
No comments:
Post a Comment