This is a link to a blog filled with practice questions and answers based on the old Additional Science specification - http://cheneygcsesciencey11.blogspot.co.uk/
We don't have so many resources for the new specification, and it hasn't change hugely, so make use of these. Bear in mind that some content has been removed from B2, C2 and P2, and some has been added. You can check exactly what you need to know here:
http://www.sciencelab.org.uk/gcses/additional-science-unit-1.php
The 'what's changed' tab shows what's gone in and come out of each topic.
Wednesday, 12 December 2012
Tuesday, 4 December 2012
New Kerboodle material
Dear 11yq, new exciting revision material has been just created in KERBOODLE for your new RADIATION topic. The material is divided in 2 parts: ONLINE TESTS and DOWNLOADABLE.
The ASSIGNMENT won't be there for ever, and I would like to have it all done BEFORE our progress test in less than 2 weeks time.
See you in the classroom!
Dr. Pontecorvi
The ASSIGNMENT won't be there for ever, and I would like to have it all done BEFORE our progress test in less than 2 weeks time.
See you in the classroom!
Dr. Pontecorvi
Wednesday, 21 November 2012
Physics trip opportunity
We have been given an exciting opportunity to take a group of students to a talk about The Higgs Boson at the Rutherford Appleton Laboratory. This event takes place on Friday 7thDecember. It will begin at 1.30am and we should be back in school by 4.00pm. It is entitled “The Higgs Boson: a weight off all our minds” and is given by Dr William Murray – from STFC and CERN. It is open to Y11 and there are only 14 places, so it is first come first served.
Please collect a letter from Ms Hamnett and return to the school shop
Chemistry work for Wednesday 21st November and homework
I'm not well enough for school today - my voice didn't survive yesterday.
The aims of today's lesson is to get you familiar with some online resources, to revise some of your earlier chemistry work so it stays fresh in your minds and to move forward with electrolysis. What you don't finish in today's lesson is your homework for Monday - please record in your planner.
Well done to those of you who completed the self-assessment assignments I set you on kerboodle recently - I have recorded your marks and will talk to you about these. I'm not sure that you can do these now if you haven't already, but if you can please do them (if not I will re-assign them later). There is some new work on Kerboodle relating to electrolysis, and a 'test yourself' on structure and bonding to see how well you remember that - be sure to go through the questions once you have submitted and read the feedback on the ones you get wrong.
If you can't get onto kerboodle email me on tla@cheney.oxon.sch.uk - remember the school code is 5166
Sunflower is another online resource which you now have individual logins for. Your username should be the same as your kerboodle one - it's surnameforename with no space, all lower case e.g. curtisadams. Your password is the same but with your tutor group's letter and number e.g. a01 for Austin 1 (no spaces again - e.g. curtisadamsa01). The school code is chen08. There are assignments set for you here too. The periodic table can be rotated to view it as a bar chart of various different properties.
Finally the questions below can be answered directly into the blog, and will be saved to a google spreadsheet for me to mark:
Have a look at the bitesize electrolysis resources for help.
The aims of today's lesson is to get you familiar with some online resources, to revise some of your earlier chemistry work so it stays fresh in your minds and to move forward with electrolysis. What you don't finish in today's lesson is your homework for Monday - please record in your planner.
Well done to those of you who completed the self-assessment assignments I set you on kerboodle recently - I have recorded your marks and will talk to you about these. I'm not sure that you can do these now if you haven't already, but if you can please do them (if not I will re-assign them later). There is some new work on Kerboodle relating to electrolysis, and a 'test yourself' on structure and bonding to see how well you remember that - be sure to go through the questions once you have submitted and read the feedback on the ones you get wrong.
If you can't get onto kerboodle email me on tla@cheney.oxon.sch.uk - remember the school code is 5166
Sunflower is another online resource which you now have individual logins for. Your username should be the same as your kerboodle one - it's surnameforename with no space, all lower case e.g. curtisadams. Your password is the same but with your tutor group's letter and number e.g. a01 for Austin 1 (no spaces again - e.g. curtisadamsa01). The school code is chen08. There are assignments set for you here too. The periodic table can be rotated to view it as a bar chart of various different properties.
Finally the questions below can be answered directly into the blog, and will be saved to a google spreadsheet for me to mark:
Have a look at the bitesize electrolysis resources for help.
Monday, 29 October 2012
Chemistry self-assessments on Kerboodle
There are now two 'test yourself' assignments waiting for you at www.kerboodle.co.uk - one for each of our recent Chemistry topics, with a due date of Wednesday 14th November.
Email me on tla@cheney.oxon.sch.uk if you can't log in.
Monday, 22 October 2012
Chemistry homework
The new chemistry homework regime involves a weekly task to be completed for Wednesday. I will generally aim to set this on Monday of week B and on Friday of week B for the following Wednesday in each case. Wednesday hand-ins mean that those of you who don't manage to finish the work can stay at the end of the lesson to receive some help with doing so!
For this week there are actually two tasks - one has 'rolled forward' from last week.
1) Produce your own annotated diagrams illustrating and explaining the effect that concentration, gas pressure and temperature have on reaction rates. The diagram I gave you last Wednesday illustrating how Collision Theory can be used to explain the effect of surface area on reaction rate can serve as inspiration.
This might also help:
http://www.bbc.co.uk/learningzone/clips/collision-theory-and-rates-of-reaction/10668.html
This is the collision simulator we looked at last week:
http://www.animatedscience.com.au/learningmodules/collision.swf
Your diagrams should show a) what effect increasing the temperature / concentration / pressure has on the reaction rate, b) how it affects the number and/or energy of collisions between particles and c) the link between these.
2) Write a method section and draw a neat results table and graph for today's practical:
Your method section should include:
For this week there are actually two tasks - one has 'rolled forward' from last week.
1) Produce your own annotated diagrams illustrating and explaining the effect that concentration, gas pressure and temperature have on reaction rates. The diagram I gave you last Wednesday illustrating how Collision Theory can be used to explain the effect of surface area on reaction rate can serve as inspiration.
This might also help:
http://www.bbc.co.uk/learningzone/clips/collision-theory-and-rates-of-reaction/10668.html
This is the collision simulator we looked at last week:
http://www.animatedscience.com.au/learningmodules/collision.swf
Your diagrams should show a) what effect increasing the temperature / concentration / pressure has on the reaction rate, b) how it affects the number and/or energy of collisions between particles and c) the link between these.
2) Write a method section and draw a neat results table and graph for today's practical:
Your method section should include:
- Your IV, DV and control variables
- The range and interval of your IV
- A list of equipment, and brief instructions for its use, including a diagram
- A risk assessment – what could go wrong (hazards), the risk of these and how this can be reduced
- Sources of error and how these could be reduced
Sunday, 7 October 2012
Physics P2:
REVISION for FORCES (p2.2)
REVISION on WORK and MOMENTUM (p2.3)
A double set of revisions is now available for the Y11yqSc group, in Kerboodle.
The set will remain available until the end of the week (Sunday the 14th of October), by which time you should have completed all the tasks requested.
Kerboodle is a useful place where to start your revision and check your skills.
Your access to the resources and the results will obviously be monitored and recorded and marked (by yourself when prompted).
A new set of revision material and a certain amount of PPT resources will be available shortly on the ELECTRICITY and CHARGE topic.
Have a re-CHARGING weekend and see you all next week!
Dr. Pontecorvi
PS I) If you cannot access Kerboodle, you must contact me at mpo@cheney.oxon.sch.uk and I will define a day and time when manually amend your accounts.
PS II) Please check whether you have also access to Physics for You book within Kerboodle website: such resources have been set for you and you should be able to use it.
REVISION for FORCES (p2.2)
REVISION on WORK and MOMENTUM (p2.3)
A double set of revisions is now available for the Y11yqSc group, in Kerboodle.
The set will remain available until the end of the week (Sunday the 14th of October), by which time you should have completed all the tasks requested.
Kerboodle is a useful place where to start your revision and check your skills.
Your access to the resources and the results will obviously be monitored and recorded and marked (by yourself when prompted).
A new set of revision material and a certain amount of PPT resources will be available shortly on the ELECTRICITY and CHARGE topic.
Have a re-CHARGING weekend and see you all next week!
Dr. Pontecorvi
PS I) If you cannot access Kerboodle, you must contact me at mpo@cheney.oxon.sch.uk and I will define a day and time when manually amend your accounts.
PS II) Please check whether you have also access to Physics for You book within Kerboodle website: such resources have been set for you and you should be able to use it.
Friday, 5 October 2012
HYDROPONICS
Your homework for next week includes finding out about hydroponics. As it happens Wikipedia has a good entry on this - see http://en.wikipedia.org/wiki/Hydroponics
Interestingly, Oxford also has a hydroponics equipment shop - see http://www.3ch.co.uk/oxford/info_42.html
Have a good weekend. Look out for lichens.
Mr B
Interestingly, Oxford also has a hydroponics equipment shop - see http://www.3ch.co.uk/oxford/info_42.html
Have a good weekend. Look out for lichens.
Mr B
Sunday, 30 September 2012
Chemistry exam practice
Apologies for not getting this homework on here sooner - some exam practice ahead of our chemistry progress test which is currently planned for Wednesday 10th October:
https://docs.google.com/open?id=0B2iNY974PgcfQk1Kc0VvUnZuT0E
For this Wednesday 3rd.
https://docs.google.com/open?id=0B2iNY974PgcfQk1Kc0VvUnZuT0E
For this Wednesday 3rd.
Friday, 28 September 2012
Sorry I missed you this Friday because of INSET. To prepare for next lesson (homework!) please do have a go at the exam style questions on cells, tissues and organs on Kerboodle. Not sure if this link will work (without you logging in) but try http://live.kerboodle.com/secondary/Course/Browse.aspx?Course=NDEyNDYyMw==&Track=True
Biology - diffusion Powerpoint file
Here's the presentation I promised you...
https://docs.google.com/open?id=0B_U0sUOJUH_CT0RUbjM0ZGJkMWM
https://docs.google.com/open?id=0B_U0sUOJUH_CT0RUbjM0ZGJkMWM
Thursday, 13 September 2012
Chemistry - % Mass and Empirical Formula Calculation
Here is some help on BBC Bitesize for these two types of calculation which we looked at yesterday:
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/chemcalc/chemcalc_bothrev5.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/chemcalc/chemcalc_higherrev2.shtml
Youtube provides a friendly science teacher to explain empirical formulae if you need further refreshment.
Your homework for Friday afternoon is to find one hard % composition question, and one hard empirical formula question from the sheets I've already given you, and have a go at these.
If you can't track down a relevant sheet, have a go at these:
1) What is the percentage of Oxygen in Na2So4?
2) What is the empirical formula of a substance that contains 18g of carbon, 4.5g of hydrogen and 12g of oxygen?
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/chemcalc/chemcalc_bothrev5.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/chemcalc/chemcalc_higherrev2.shtml
Youtube provides a friendly science teacher to explain empirical formulae if you need further refreshment.
Your homework for Friday afternoon is to find one hard % composition question, and one hard empirical formula question from the sheets I've already given you, and have a go at these.
If you can't track down a relevant sheet, have a go at these:
1) What is the percentage of Oxygen in Na2So4?
2) What is the empirical formula of a substance that contains 18g of carbon, 4.5g of hydrogen and 12g of oxygen?
Wednesday, 18 July 2012
Tuesday, 17 July 2012
Well done to the few present today who, without almost ANY indication, have devised an experiment to show the conservation of momentum, using an air track, data-logger, light gates and their brains. The same group will interact with me during the summer in order to get a nice and clear METHOD done and ready to be printed. They will also stage the demo again in September for the rest of the class, which will be left to do some more calculations and explaining what they are observing.
Next lesson (tomorrow Wednesday the 18th of July, p4) is the last lesson and we might be talking about why we do science, seeing a short movie about that etc...
Next lesson (tomorrow Wednesday the 18th of July, p4) is the last lesson and we might be talking about why we do science, seeing a short movie about that etc...
Monday, 16 July 2012
Mole Calculations
On Friday we practised balancing equations and working out formula masses. This will allow us to calculate the number of moles, and the masses, of reactants and products using the equations:
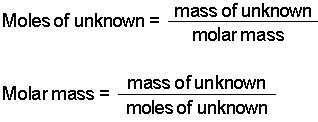

Here is an example which we will go through at the start of the lesson, and which will be easy for you if you've read this first:
What mass of calcium hycroxide do we need to neutralise 146g of hydrochloric acid?
First we need a balanced symbol equation:
2HCl + Ca(OH)2 > CaCl2 + 2H2O
This tells us that one mole of calcium hydroxide will react with two moles of HCl (because the balanced equation has 2HCl but only one Ca(OH)2 )
So how many moles of HCl do we have? This is the mass divided by the molar mass, so 146 / (1+35/5) = 4 mol.
So how many moles of Ca(OH)2 do we need? One will react with two moles of HCl, so we will need 2 to react with 4.
What is the mass of Ca(OH)2 needed? This is given by mass = moles x formula mass = 2 x (40+(2 x (16+1))) = 2 x 74 = 148g
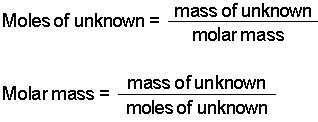

Here is an example which we will go through at the start of the lesson, and which will be easy for you if you've read this first:
What mass of calcium hycroxide do we need to neutralise 146g of hydrochloric acid?
First we need a balanced symbol equation:
2HCl + Ca(OH)2 > CaCl2 + 2H2O
This tells us that one mole of calcium hydroxide will react with two moles of HCl (because the balanced equation has 2HCl but only one Ca(OH)2 )
So how many moles of HCl do we have? This is the mass divided by the molar mass, so 146 / (1+35/5) = 4 mol.
So how many moles of Ca(OH)2 do we need? One will react with two moles of HCl, so we will need 2 to react with 4.
What is the mass of Ca(OH)2 needed? This is given by mass = moles x formula mass = 2 x (40+(2 x (16+1))) = 2 x 74 = 148g
Thursday, 12 July 2012
Ph2 Physics - Momentum and its Conservation!
Lesson 2: Wednesday 11/07/2012 - p3/W8
Today you will work in group to create a presentation of the following:
- State how is the momentum before and after an explosion
- Describe what does cause the recoil when firing a bullet
- Apply the PCM to calculate how two objects recoil from each other after an explosion
Tuesday, 10 July 2012
Ph2 Physics - Momentum and its Conservation!
Lesson 1: Wednesday 11/07/2012 - p3/W8
From today's lesson you need be able to do the following:
- Calculate the momentum of an object
- State the Principle of Conservation of Momentum
- Apply the PCM to every day situations
- Describe and explain some examples about where, in everyday life, the Conservation of Momentum plays a vital role..
Monday, 9 July 2012
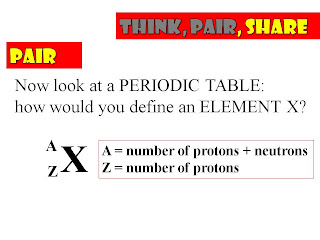
CH2 Chemistry - Mass of Atoms
From today's lesson you need be able to do the following:
- State what an atom’s atomic number is.
- State what an atom’s mass number is.
- Know what the relative masses of protons, neutrons and electrons are.
- Explain what an isotope is.
- Describe and explain the physical and chemical properties of isotopes of the same element.
Then watch this one, about Mendeleev's construction of the periodic table based on the Atomic Weights of the elements known at the time - there will be a couple of quick test questions on this on Wednesday.
Subscribe to:
Comments (Atom)